
p
<
0.001;
Fig. 2B) compared with IDC/CA– tumors,
concurring with an increased proportion of chromothriptic
tumors (34.2% vs 16.0%,
p
= 0.033; Supplementary Fig. 4).
Consistent with our previous findings
[5,6], PGA was an
independent predictor of biochemical and metastatic
relapses on multivariable analyses (Supplementary
Table 5). In multivariable models that considered both
IDC/CA and PGA, IDC/CA and PGA demonstrated comparable
prognostic capabilities
( Tables 1 and 2 ,and Supplementary
Table 4). When combined, these genomic–pathological
indices offered further clinical stratification over IDC/CA or
PGA alone. Against the reference subgroup of low PGA, IDC/
CA– tumors, patients with high PGA, IDC/CA+ tumors
harbored the greatest risks of biochemical relapse (HR
3.3 [95% CI = 2.1–5.0],
p
<
0.0001) and metastasis (HR
5.5 [95% CI = 2.5–12.2],
p
<
0.0001) compared with the
other subgroups of low PGA, IDC/CA+ tumors, and high PGA,
IDC/CA– tumors
( Fig. 2 C). Additionally, combinatorial
clinical + IDC/CA + PGA model demonstrated the most
superior discrimination for biochemical relapse and metas-
tasis relative to the clinical only and clinical + IDC/CA
models (see Harrell’s C-index in
Tables 1 and 2 ).
Given the presence of genomic instability within IDC/
CA+ prostate cancers, we asked whether such tumors had a
specific RNA expression profile (using 63 IDC/CA+ and
93 IDC/CA– tumors from the Canadian cohort). Remarkably,
despite testing for
>
[20_TD$DIFF]
25,000 genes, IDC/CA+ tumors
expressed only one gene that was
>
3-fold higher than
IDC/CA– tumors: S
ChLAP1
, a long noncoding RNA previously
associated with adverse prognosis following prostatectomy
[20–22](fold change = 3.23, false discovery rate-corrected
p
<
0.0001,
Fig. 3 Aand 3B).
SChLAP1
expression in dissected
tumor specimens yielded an area under the curve value of
0.74 (95% CI = 0.65–0.82; continuous rawmRNA abundance
values) for the detection of IDC/CA. This association was
corroborated by
SChLAP1
RNA-ISH in 393 prostatectomy
specimens (EMC cohort), showing a significant association
and an overall accuracy of 82.4% for
SChLAP1
expression
within IDC/CA+ tumors (
p
<
0.001;
Fig. 3C and Supplemen-
tary Fig. 5). Interestingly, we observed diffuse
SChLAP1
RNA-
ISH signals in both IDC/CA and adjacent glandular
adenocarcinoma in some of the TMA cores
( Fig. 3 D). Finally,
biochemical relapse was significantly increased only in the
SChLAP1
+, IDC/CA+ subgroup (HR 2.6 [95% CI = 1.4–4.7],
p
= 0.0027;
Fig. 3 E); independent of PGA (median of 6.79,
SChLAP1
+, IDC/CA+ tumors vs 5.95,
SChLAP1
–, IDC/CA+
tumors;
p
= 0.18). Taken together, our findings support
‘‘aggression’’ field-wide alterations in IDC/CA+ prostate
cancers that are associated with genomic instability and
specific gene expression in situ for
SChLAP1
.
4.
Discussion
IDC and CA subpathologies predict for biochemical relapse
and metastasis following treatment of localized prostate
cancer (this study and references
[7–[6_TD$DIFF]
9,23–[7_TD$DIFF]
25] ). Our data
suggest that several aggressive features exist within
prostate cancers that are associated with lethal IDC and
[(Fig._1)TD$FIG]
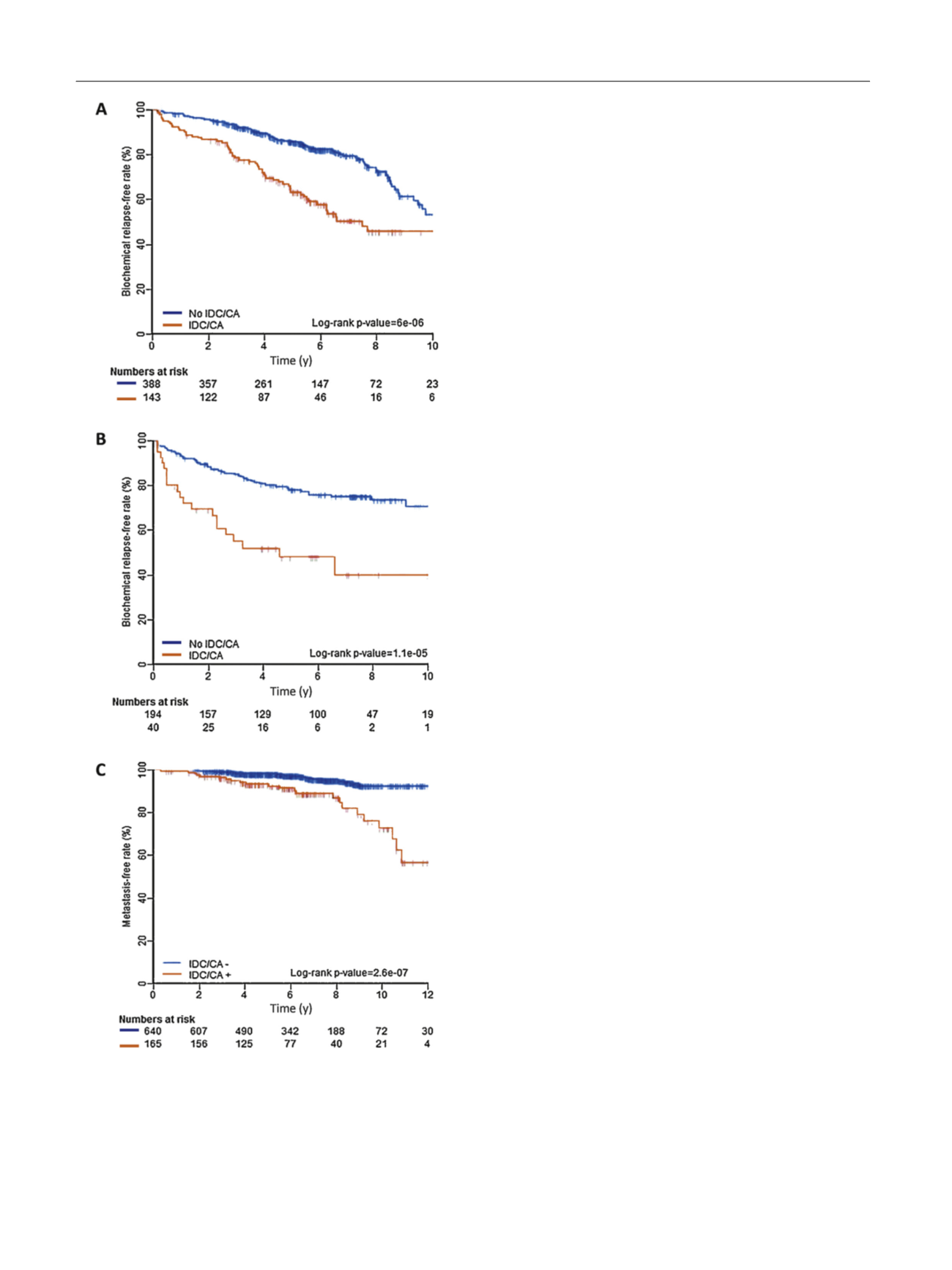
Fig. 1 – Clinical outcomes stratified by intraductal carcinoma (IDC) and
cribriform architecture (CA). Biochemical relapse-free rates for (A)
Canadian, and (B) MSKCC cohorts. (C) Metastasis-free rates using pooled
data.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 6 6 5 – 6 7 4
668
















