
grading systems. In addition, four pathologists (A + B + C + D)
reviewed the slides for the 2004/2016WHO classification on
two separate occasions. May et al
[33]reported reproduc-
ibility of both grading systems between four independent
pathologists
( Table 1).
3.3.
RoB and confounding assessment of the included studies
Figure 3presents the RoB summary for the 20 included
trials
[3,16–34]. We found the highest RoB in study attrition
(incomplete outcome data), study confounders (validity,
reliability, and similarity of measurement), and study
participation (representativeness of the study sample)
[10] .The risk of reporting bias (selective reporting) was
high in less than one-third of studies. The risks of bias in
prognostic factor (tumour grade) measurement and out-
come measurement (adequacy of outcome measurement)
were low.
For the three most important prognostic confounders,
tumour stage was well described, but presence of CIS and
use of adjuvant treatment were incompletely reported
( Table 1). Therefore, it was difficult to factor these last two
confounders into the analyses. Some subgroup analyses
were performed in Ta and T1 patients
( Tables 2 and 3 ).
3.4.
Comparisons of prognostic outcome measures
For analysis of progression, recurrence, and overall
and cancer specific survival, most available information
concerned the number of patients with an event during
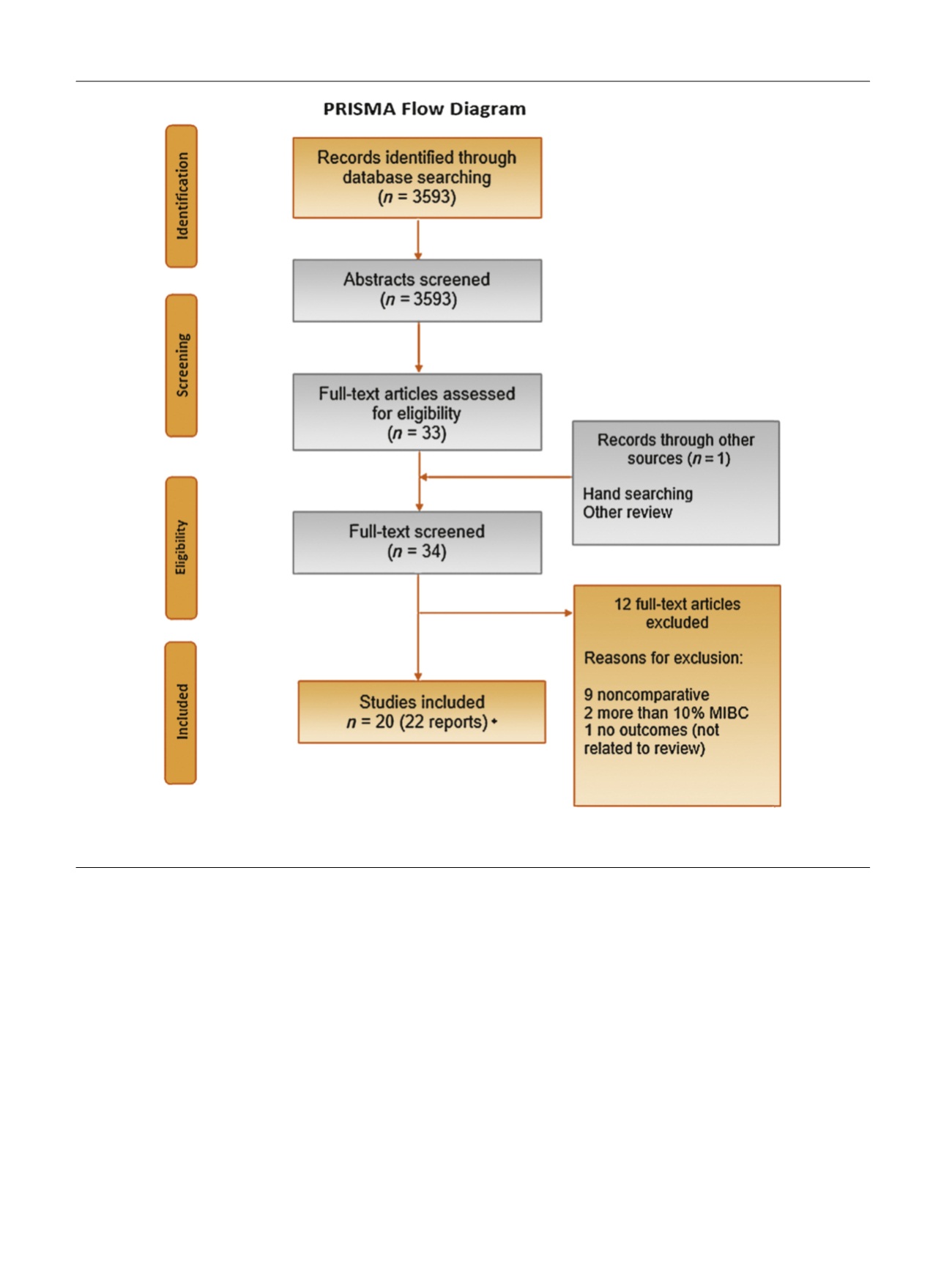
[(Fig._2)TD$FIG]
Fig. 2 – PRISMA diagram (applicable for both prognostic and reproducibility reviews). MIBC = muscle-invasive bladder cancer; PRISMA = Preferred
Reporting Items for Systematic Reviews and Meta-analysis. * = Three of those studies were also eligible for the reproducibility part.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 8 0 1 – 8 1 3
804
















