
Recruiters were from urological cancer trials
[13,23,25, 27,28,40,41,44,55], other cancer trials
[24,27,28,32,35,40,44, 49,50,52,54,55], mental health
[27, 28, 39,46,50,55], ortho-
paedics
[31,33,38,49,56], diabetes
[36,47] ,vascular surgery
[40,49], peripartum trials
[37,53], HIV
[30], smoking
cessation
[42], and pressure area care
[51] .The majority
of studies (32/35) focused on RCTs that were conducted in
the UK
[13,24–29,31–41,43–56].
Studies consisted mostly of interviews or focus groups
with those involved in recruitment to RCTs
[13,23– 37,39,42–44,46–56], sometimes alongside interviews with
patients who had been offered the opportunity to join the
trial
[23–26,33,37]. Several studies audio recorded con-
sultations where recruiters discussed the RCT with eligible
patients
[13,24–26,31,32,38,40,41,44,45,49,50,52,55][10_TD$DIFF]
.A
summary of the included studies is shown in
Table 2.
3.2.
Part 1: why is recruitment so challenging?
3.2.1.
Exploring facilitators and barriers to recruitment
Overall, interviews with healthcare professionals highlight-
ed a number of positive aspects of being involved in
research. For instance, recruiters described how intellectual
challenges and professional kudos were incentives to
participate in RCTs
[30,43]. Many felt that participation
in trials was beneficial in that it provided patients with
access to novel treatments
[29,30,34,39,43,51] ,gave
patients hope
[29,30], and monitored participants closely
[30,39,43].
The majority of studies used interviews to understand
recruiters’ perceptions of factors that impacted upon
recruitment to RCTs. Many described how collaboration
within the clinical team was vital
[29,34,35,51,54]. Aware-
ness and understanding of the particular RCT was also
deemed to be important
[29,33,39,42,43,54], particularly in
terms of the eligibility criteria
[34,35,39]and study
processes
[35,36,39]. Many recruiters felt that receiving
regular updates and feedback from the trial team was
beneficial
[34,35,47], although some found this overwhelm-
ing
[36] .[11_TD$DIFF]
Recruiters highlighted a range of logistical and practical
issues that had made recruitment challenging
[12_TD$DIFF]
( Table 1 ).
Many commented on a lack of eligible patients
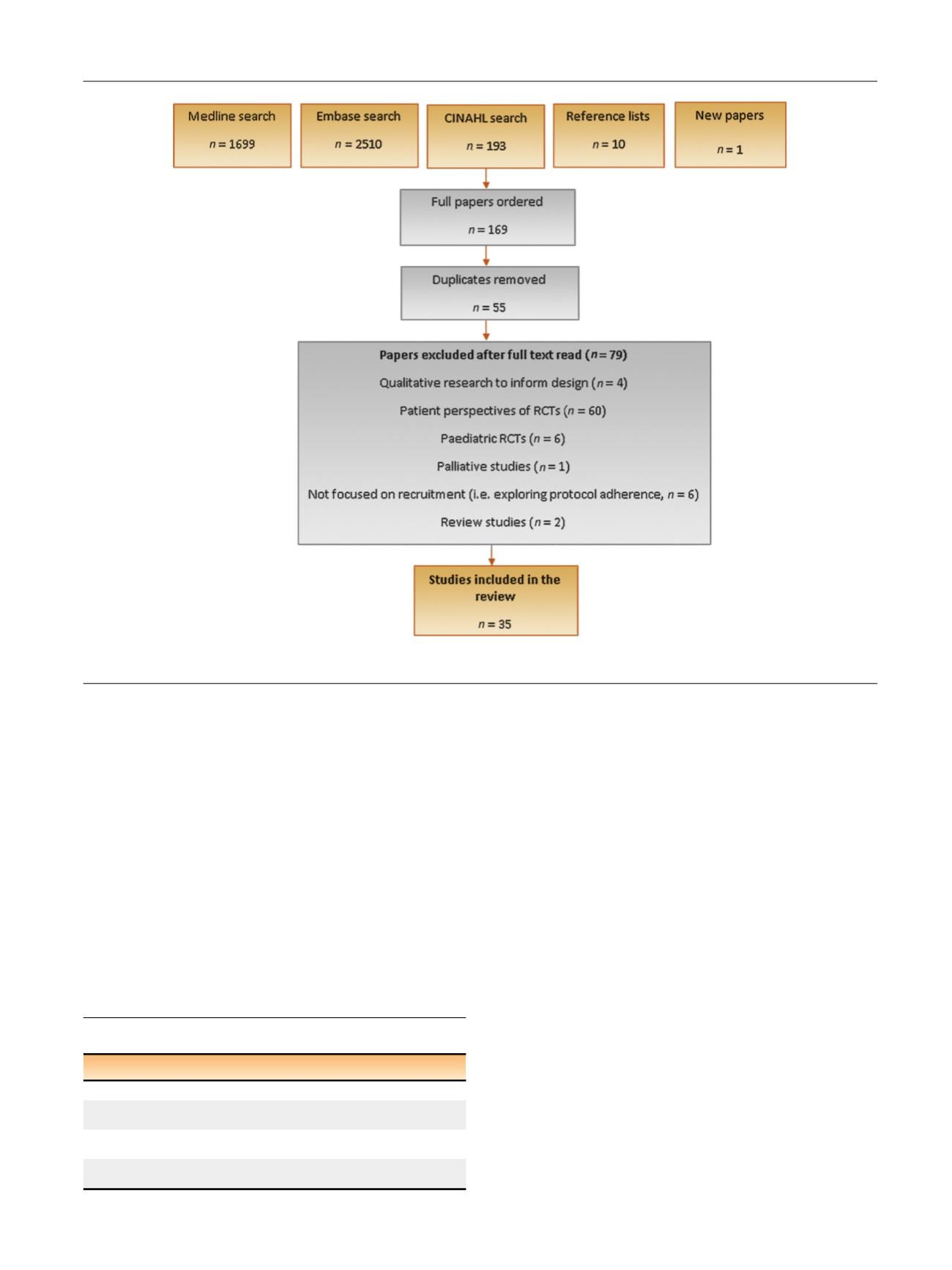
[(Fig._1)TD$FIG]
Fig. 1 – Steps of the literature search and selection process of articles. RCT = randomised controlled trial.
Table 1 – Commonly reported barriers to recruitment
Recruitment issue
References
Lack of eligible patients
[24,27,36,44,52,53]Patients dislike concept of
randomisation
[24,25,27,35,50,52]Patients express strong preferences
for a particular treatment
[23–25,32,35,44,52,53]Lack of clinician time for
research activities
[23,29,33,34,36,39,42,43,46,47,51,53]E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 7 8 9 – 7 9 8
791
















