
than patients who underwent DE-EBRT alone. Despite this
potential benefit, we found that the majority of men in this
cohort were treated without LDR-B boost, and that use of
LDR-B boost in this population declined across the study
period to a low of
<
15% in 2012. These findings complement
results from large randomized trials, supporting the efficacy
of LDR-B boost and suggesting that improvements in
biochemical control may translate to better OS with
additional follow-up.
The ASCENDE-RT trial recently analyzed 400 patients
with unfavorable PCa and demonstrated a 53% improve-
ment in biochemical failure in favor of LDR-B. At median
follow-up of 6.5 yr, there was no significant difference in
7-yr OS, but the trend did favor LDR-B boost (85.7% vs
81.5%). However, the study was underpowered and median
survival had not been reached, estimated to occur at
approximately 13 yr
[21]. Other retrospective analyses
evaluating different treatment modalities and patient
populations are in agreement with the primary findings
of this study. A recent analysis by Amini et al
[25]showed
that EBRT plus brachytherapy was associated with better
5-yr OS for both intermediate- and high-risk disease (85% vs
91%;
p
<
0.001). However, this benefit was no longer seen
on exclusion of patients who received
<
79.2 Gy. In the
present study, limited to LDR-B boost, using a more recent
cohort and controlling for the timing of AS, we found a
similar 7-yr OS improvement associated with brachythera-
py boost (82% vs 73%;
p
<
0.001). The benefit of which
remained regardless of dose. This is an important finding,
as doses 79.2 Gy are considered standard for modern
DE-EBRT.
In this analysis, the benefit of LDR-B boost persisted on
MVA and after adjusting for known confounders in a
propensity score–matched analysis. However, many of the
deaths seen in this study probably represent non-PCa
deaths, as demonstrated by early differences in OS. To
account for unknown confounders and in an attempt to
exclude non-PCa deaths, we created a novel analysis by
selecting only men younger than 60 yr with no comorbid-
ities. The validity of this analysis relies on the accuracy of
the comorbidity assessment in the NCDB, which has been
validated as being similar to Surveillance, Epidemiology,
and End Results (SEER)-Medicare index claims
[26]. This
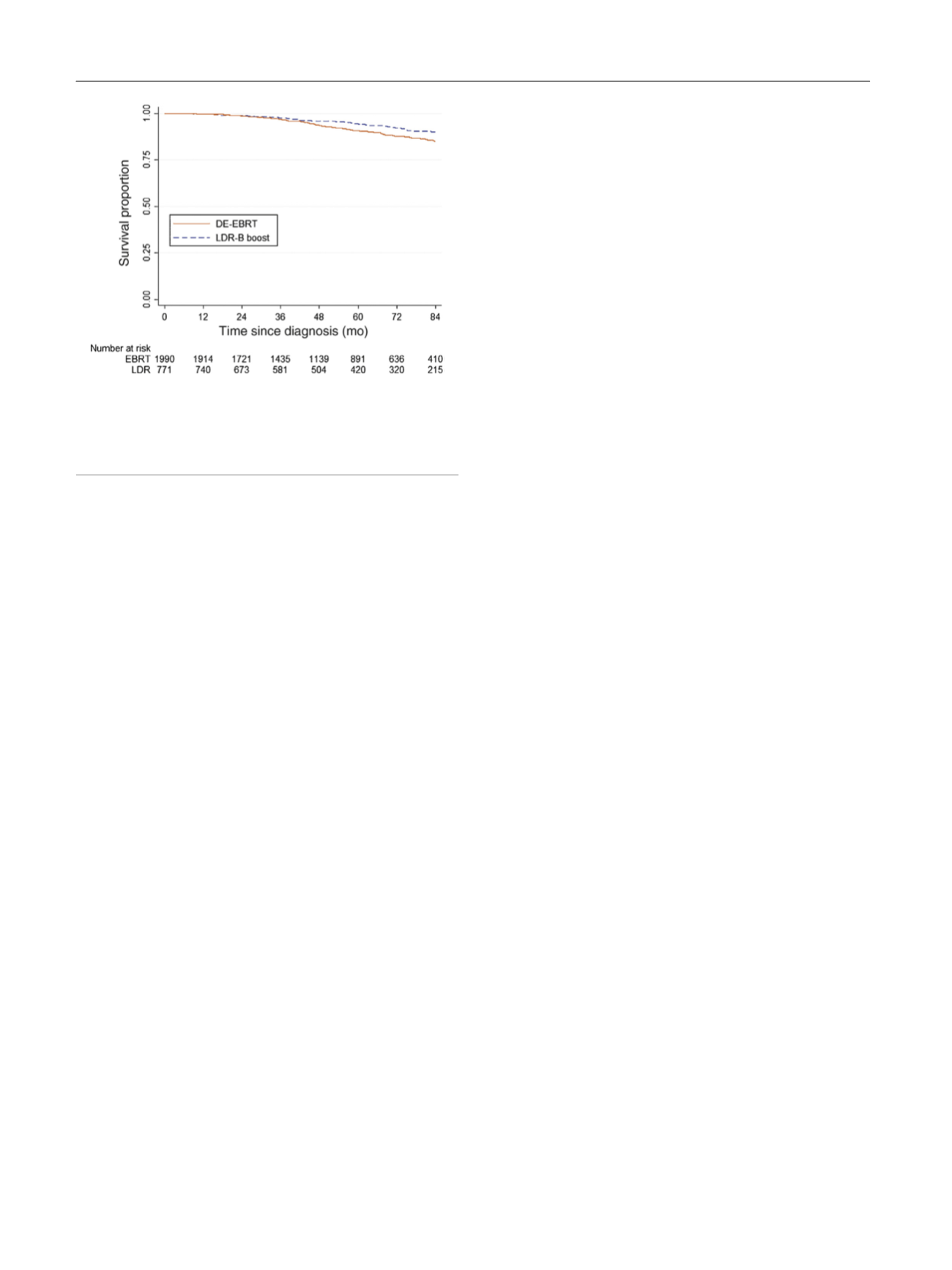
showed that the benefit of LDR-B persisted and was most
pronounced after 5 yr (7-yr OS 90% vs 85%), with an absolute
OS difference similar to that seen at 7 yr in the ASCENDE-RT
trial. Other studies have suggested PCa-specific mortality
(PCSM) of between 5% and 10% at 7 yr for patients with
unfavorable intermediate- and high-risk disease treated
with ERBT and hormone therapy
[27] .A recent SEER
database study by Muralidhar et al
[28]evaluated PCSM
among 45 078 patients with intermediate and high-risk PCa
who received either EBRT alone or EBRT and brachytherapy
boost at either a high-dose rate (HDR) or LDR. They found a
significant benefit in PCSM for patients receiving EBRT and
brachytherapy compared to EBRT alone (3.9% vs 5.3%;
p
= 0.02) for patients with high-risk disease. However, this
benefit did not persist when evaluating those with
intermediate-risk disease or favorable high-risk disease.
Of note, the median follow-up was only 3.6 yr in the
favorable high-risk cohort, which may have contributed to
the lack of a demonstrable survival benefit owing to the
long natural history of PCa. In contrast, our analysis
suggested that the associated improvement in OS for
patients selected for LDR-B boost persisted, as there was
no interaction between risk group and treatment.
Interestingly, despite improved outcomes for LDR-B
boost, our patterns of care analysis suggest a decrease in
LDR-B boost utilization between 2004 and 2012
( Fig. 1).
This confirms a previous report that showed declines in the
use of combined EBRT and brachytherapy boost between
2004 and 2009
[29]. Past investigators have proposed
multiple reasons for the decline, including an increase in the
number of prostatectomies
[29], increases in reimburse-
ment for other radiation modalities including intensity-
modulated radiation
[30] ,a decrease in brachytherapy
training
[31], and perception of brachytherapy as a
procedure with excessive liability risk
[32].
There are several limitations to our study. First, we were
not able to compare treatment toxicity in the NCDB, and any
potential benefits of a treatment modality should be
weighed against the possibility of harm. The ASCENDE-RT
trial demonstrated that a significantly larger proportion of
patients who received LDR-B boost experienced late severe-
grade ( 3) urinary side effects compared to patients who
received EBRT alone (8% vs 2%)
[33]. Second, given the
retrospective nature, data collection limitations are inher-
ent. Reporting bias and treatment facility selection bias may
exist, as only CoC-accredited hospitals contribute data to
the NCDB, although the NCDB still captures 70% of newly
diagnosed malignancies and has extensive quality assur-
ance mechanisms in place to ensure accurate data capture.
In addition, nonrandomized comparisons can lead to false
conclusions. It is likely that bias was introduced by
clinicians in selecting patients to undergo LDR-B that we
could not control for, despite our best efforts, which
[(Fig._3)TD$FIG]
Fig. 3 – Kaplan-Meier survival curves for men aged <60 yr with no
comorbidities and stratified by treatment type with either dose-
escalated external beam radiation therapy (DE-EBRT) or low-dose-rate
brachytherapy (LDR-B) boost (7-yr overall survival 85% vs 90%;
p
< 0.001).
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 7 3 8 – 7 4 4
742
















