
developed a priori: age, sex, description of primary
pathology, severity, or bother of nocturia.
Studies are summarized in
Figure 1 .3.
Evidence synthesis
3.1.
Conservative management
One study investigated a behavioral modification program
with desmopressin in comparison with desmopressin
monotherapy in patients with nocturnal polyuria and
nocturia ( 2 voids/night; Levels of Evidence [LoE] 1b)
[8]. Nocturnal voids declined by –1.5 with combined
therapy, compared with –1.2 on desmopressin alone (not
significant). Another group randomized obese men with
LUTS already on tamsulosin to receive a basic or a
comprehensive weight reduction program (LoE 2b)
[9]. The improvement in nocturia episodes was similar in
both arms (–0.1 0.9). Both studies showed mild adverse
events only in pharmacotherapy-related arms.
3.2.
Antidiuretic therapy
The antidiuretic hormone arginine vasopressin increases
renal water reabsorption and urinary osmolality. Antidi-
uretic therapy using the arginine vasopressin V2 receptor
agonist desmopressin, with dose titration to achieve the
best clinical response (as defined by the researchers in each
study; including the dose to achieve either no voids per
night, a decrease in nocturnal urine production of 20%, or
nocturnal diuresis
<
0.5 ml/min), was more effective than
placebo in terms of reduced nocturnal voiding frequency
( Table 1) and duration of undisturbed sleep
( Table 2).
An RCT evaluated desmopressin (0.1 mg, 0.2 mg, or
0.4 mg, escalated according to response) in adults with 2
voids/night (LoE 1b)
[10] .One hundred and twenty-seven
patients (85 men) achieving
>
20% reduction in nocturnal
diuresis entered a double-blind efficacy phase. More
desmopressin-treated patients showed
>
50% reduction in
nocturia (33% vs 11%), reduced mean number of nocturnal
voids (39% vs 15%; absolute difference –0.84 voids/night),
and duration of the first sleep.
Adult men (
n
= 151) with 2 voids/night were studied
for 3 wk, following a dose titration phase (LoE 1b)
[11]. Nocturnal voids decreased from 3.0 to 1.7 on
desmopressin (vs 3.2–2.7 on placebo), with 34% and 3%
experiencing fewer than half the number of nocturnal voids,
respectively. Mean duration of the first sleep period
increased from 2.7 h to 4.5 h (vs 2.5–2.9 h). A fall in serum
sodium level to
<
130 mmol/l was seen in 4% of patients. In a
small short-term crossover study incorporating a dose-
response titration, desmopressin was associated with a
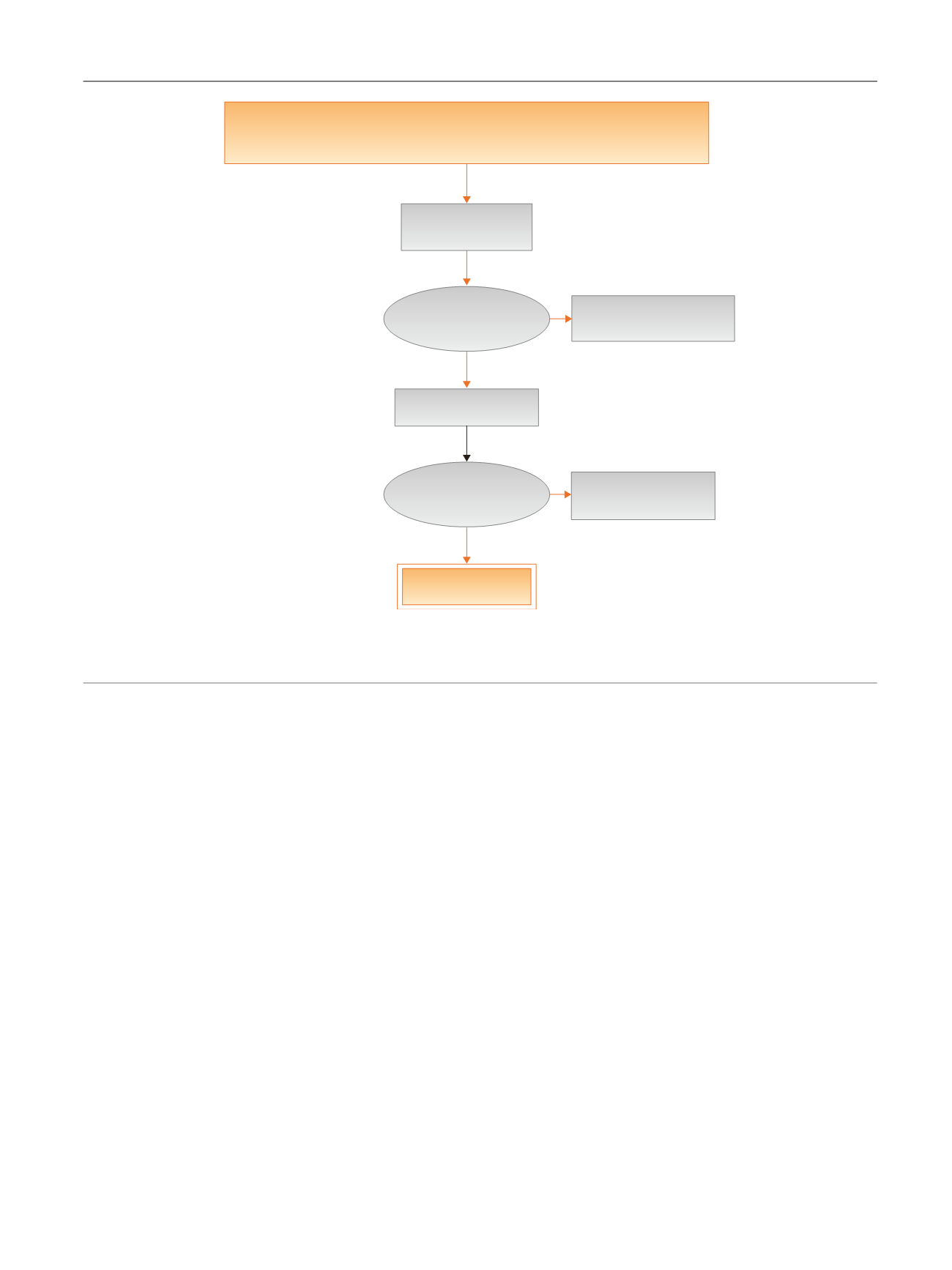
[(Fig._1)TD$FIG]
Embase, Medline, Cochrane SRs, Cochrane Central (Cochrane HTA, DARE, HEED)
No date restriction
3554 Citation(s)
3519 Nonduplicate
citations screened
Inclusion/exclusion
criteria applied
3376 Articles excluded
after title/abstract screen
143 Articles retrieved
Inclusion/exclusion
criteria applied
99 Articles excluded
after full-text screen
44 Articles included
Fig. 1 – Systematic review flow chart.
DARE = Database of Abstracts of Reviews of Effects; HEED = Health Economic Evaluations Database; HTA = Health Technology Assessment; SR = systemic
reviews.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 7 5 7 – 7 6 9
759
















