
 [47]
.
[47]
.
The group receiving the combination including
nonsteroidal anti-inflammatory drugs (NSAID) experienced
a greater reduction in nocturia (–1.5 0.9 vs –1.1 0.9,
p
= 0.034), but with a higher incidence of gastrointestinal side
effects. Gastric discomfort with loxoprofen was seen in 12.5%,
as compared with 2.6% of the control group.
3.4.3.
Phytotherapy
An 8-wk placebo-controlled RCT looked at SagaPro (Saga-
Medica, Reykjavik, Iceland), a product derived from
Angelica
archangelica
leaf, in 69 men with 2 nocturnal voids (LoE
1b)
[48]. The study found no significant difference between
the treatment groups, except on post-hoc subgroup
analysis. In a crossover trial of furosemide and gosha-
jinki-gan, 36 patients were reported to have improved
symptom score, quality of life, nocturnal frequency, and
hours of undisturbed sleep with both medications (LoE 2b)
[49] .3.4.4.
Agents to promote sleep
A crossover RCT of 20men with bladder outflow obstruction
and nocturia (mean 3.1/night) compared 2 mg melatonin
with placebo (LoE 1b)
[50]. Melatonin and placebo caused a
decrease in nocturia of 0.32 and 0.05 episodes/night
(
p
= 0.07). Nocturia responder rates (a mean reduction of
at least –0.5 episodes/night) were higher with active
medication (
p
= 0.04). Daytime urinary frequency and IPSS
were minimally altered.
3.4.5.
Summary of medical therapy of nocturia in men
A summary of the effect of medical therapy, excluding
antidiuretic, on nocturia is given in
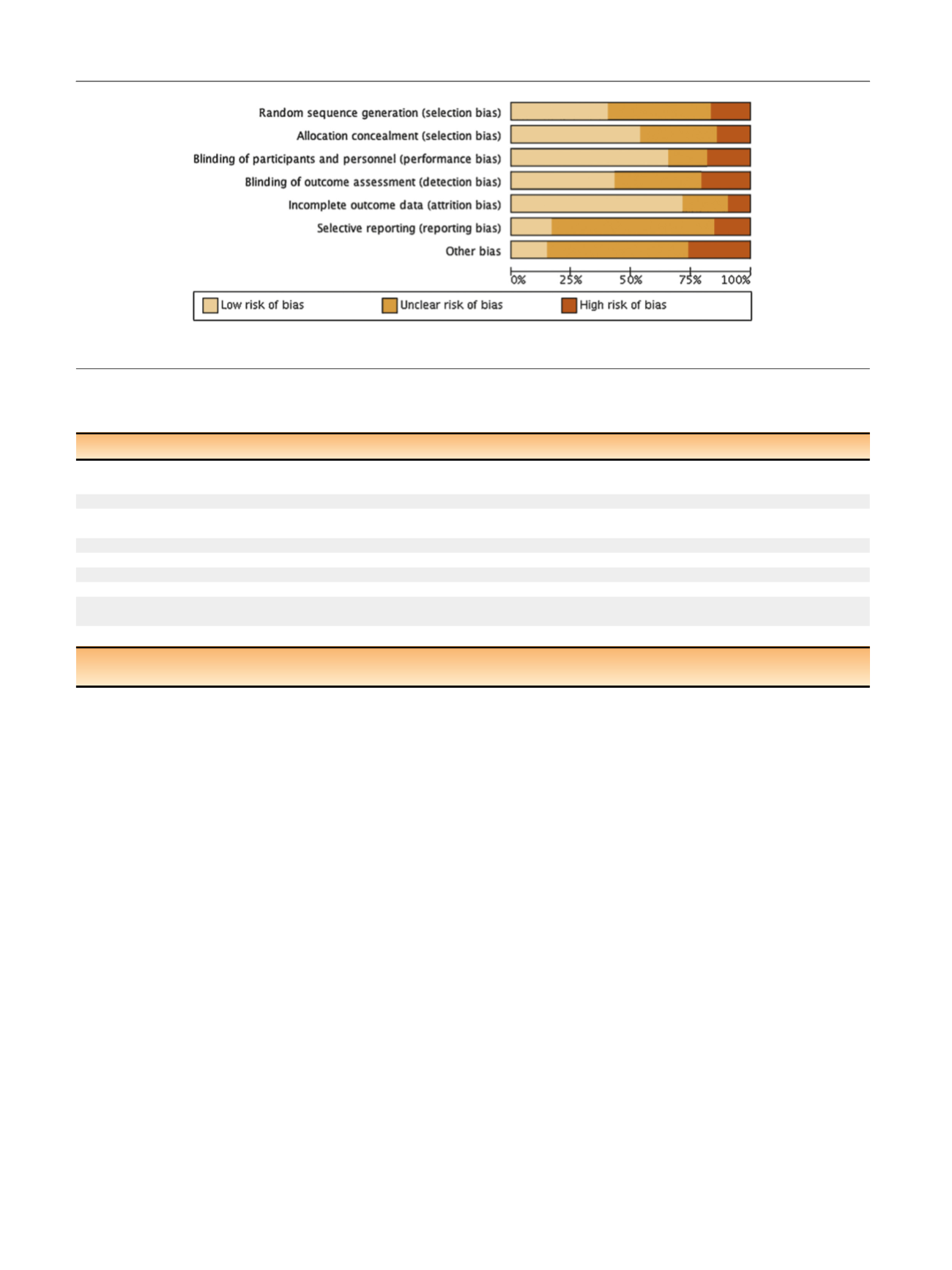
Table 5. Risk of bias
summary for all treatments is given in
Figure 2, and a table
for individual studies is given in Supplementary data.
Recommendations from the European Association of
Urology Guidelines panel for Nonneurogenic Male LUTS
are given in
Table 6.
4.
Conclusions
A detailed review of principal medical therapies of nocturia
has been presented. It assumes that conservative measures
are addressed as part of the care package. The nature of the
reports included reflects the importance of several influ-
ences; the use of subjective or objective markers as primary
outcomes, the range of severity included (twice per night
being a commonly applied threshold), and the importance
of subjective ‘‘bother.’’ The differences apparent in pub-
lished literature in regards to populations, definitions, study
design, outcome measures, and treatment durations repre-
sent a substantial limitation for the evidence base of
nocturia therapy. In nocturia, some trials report an
Table 6 – European Association of Urology Guidelines Panel for Non-neurogenic Male Lower Urinary Tract Symptoms (LUTS)
recommendations on medication treatment of nocturia in men
LE GR
Treatment should aim to address underlying causative factors, which may be behavioral, systemic condition(s), sleep disorders, lower urinary
tract dysfunction, or a combination of factors
4
A aLifestyle changes to reduce nocturnal urine volume and episodes of nocturia, and improve sleep quality should be discussed with the patient
3
A aDesmopressin may be prescribed to decrease nocturia in men under the age of 65 yr. Screening for hyponatremia must be undertaken at
baseline, during dose titration and during treatment
1a
A
a
-1 adrenergic antagonists may be offered to men with nocturia associated with lower urinary tract symptoms
1b B
Antimuscarinic drugs may be offered to men with nocturia associated with overactive bladder
1b B
5
a
-Reductase inhibitors may be offered to men with nocturia who have moderate-to-severe LUTS and an enlarged prostate (
>
40 ml)
1b C
PDE5 inhibitors should not be offered for the treatment of nocturia
1b B
A trial of timed diuretic therapy may be offered to men with nocturia due to nocturnal polyuria. Screening for hyponatremia should be
undertaken at baseline and during treatment
1b C
Agents to promote sleep may be used to aid return to sleep in men with nocturia
2
C
GR = grade; LE = level of evidence; PDE5 = phosphodiesterase type 5.
a
Upgraded based on panel consensus.
[(Fig._2)TD$FIG]
Fig. 2 – Risk of bias.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 7 5 7 – 7 6 9
766
















